Simple, straightforward logistics
We’ll handle the logistics and device management so you can focus on your patients.
Simple, straightforward logistics
We’ll handle the logistics and device management so you can focus on your patients.
Integrated and intuitive distribution and retrieval
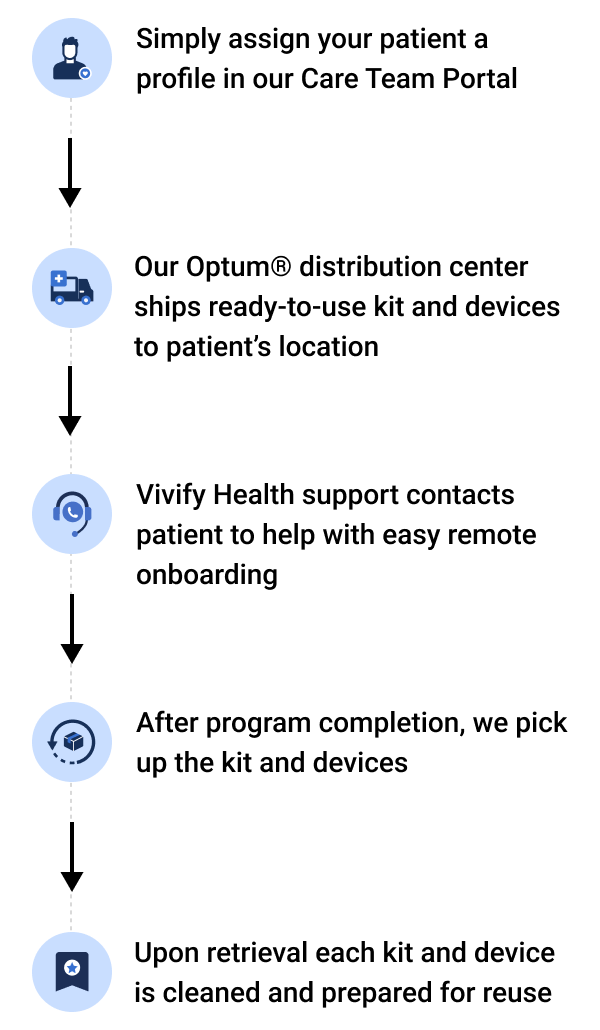
To begin the distribution process, simply assign a patient to a +HomeTM or +GoTM program in the Care Team Portal. Vivify Health takes it from there, from patient onboarding to retrieval. Through the portal, your care team always has access to shipment tracking, shipping information editing, and logistics reports.

Kit and device management options

Kit-to-patient
Includes storing and shipping it directly to the patient upon order receipt.

Kit-to-customer
Includes kits sent to your site for distribution to your patients. After patients complete their remote patient monitoring programs, the kits are retrieved and returned to our facility for reprocessing.

Common inventory
A hardware-as-a-service (HaaS) program utilizing hardware procured and maintained by Vivify Health. We take responsibility for keeping hardware up to date and replenishing/replacing it at the end of life.
End-to-end device management
From the Optum-owned distribution center, our teams procure, validation test, sanitize, ship, reprocess, and assemble health kits. FDA-registered and Joint Commission accredited, our U.S.-based facility handles Class I-III medical devices, maintaining a master record of traceability.
Our full end-to-end logistics services include:
Original equipment manufacturer(OEM) medical device receiving, testing, and cleaning to manufacturer's specifications
Kit build and packaging, including unique device identification (UDI) for tracking and traceability
Battery replacement
Roundtrip shipping and patient outreach to confirm pickup timeframes
Logistics messaging notifications accessible via Care Team Portal
Patient-assisted self-installation and training
End-to-end device management
From the Optum-owned, U.S.-based, FDA-registered and Joint Commission accredited distribution center, our teams procure, assemble, ship, receive, clean and prepare kits for re-use.
Our full end-to-end logistics services include:
Original equipment manufacturer (OEM) device receiving, testing, and cleaning to manufacturer's specifications
Kit build and packaging, including unique device identification (UDI) for tracking and traceability
Battery replacement
Roundtrip shipping and patient outreach to confirm pickup time frames
Logistics messaging notifications accessible via Care Team Portal
Patient-assisted self-installation and training
Have a large order? No problem. We’re equipped to handle requests of any size.
End-to-end device management
From the Optum-owned distribution center, our teams procure, validation test, sanitize, ship, reprocess, and assemble health kits. FDA-registered and Joint Commission accredited, our U.S.-based facility handles Class I-III medical devices, maintaining a master record of traceability.
Our full end-to-end logistics services include:
Original equipment manufacturer medical device receiving, testing, and cleaning to manufacturer's specifications
Kit build and packaging, including unique device identification (UDI) for tracking and traceability
Battery replacement
Roundtrip shipping and patient outreach to confirm pickup timeframes
Logistics messaging notifications accessible via Care Team Portal
Patient-assisted self-installation and training
Put your organization on the digital pathway to collaborative care
Find out how partnering with Vivify Health can enable you to build strong connections with your patient population.
